By: Dr Ali Raza Tunio
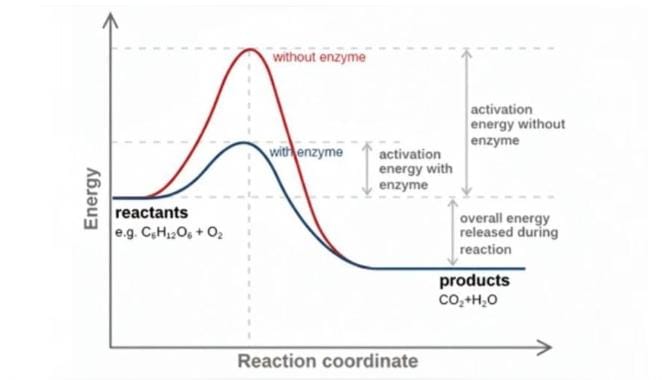
Gibbs Free Energy (ΔG) represents the portion of a reaction’s energy that can actually be used to perform work, such as building molecules or pumping ions across membranes. It is determined by the equation ΔG = ΔH – TΔS, where ΔH is the total energy of the system, TΔS is the energy lost due to entropy, and ΔG is the usable energy left to drive the reaction. When ΔG is negative (ΔG < 0), the reaction occurs spontaneously, while a positive value (ΔG > 0) means the reaction is non-spontaneous. Increasing entropy (higher ΔS) makes ΔG more negative and therefore makes the reaction more favorable. Importantly, enzymes do not change ΔG; instead, they speed up reactions by lowering the activation energy and stabilizing the transition state, allowing the reaction to proceed more quickly without altering the overall energy difference.
The Transition State Theory states that during a chemical reaction, reactant molecules pass through a high-energy, unstable intermediate state called the transition state (or activated complex),In which an unstable compound is formed and the rate of the reaction is proportional to the concentration of this transition state.”
Enzyme catalysis
In an enzymatic reaction, the reactants A and B first bind to the enzyme, forming an enzyme-substrate complex that holds them in the correct orientation for effective collisions among reactive species that proceeds reaction. From this complex, the molecules proceed to the transition state, a high-energy, unstable intermediate in which bonds are partially broken and partially formed. The enzyme stabilizes this transition state, lowering the activation energy and allowing the reaction to proceed faster. Finally, the reaction completes and the products C and D are released, while the enzyme itself remains unchanged and ready to catalyze another reaction.
Enzymes do not increase kinetic energy they lower the activation energy and propers the orientation of reactants so that the molecules existing kinetic energy is sufficient for effective collisions to reach the transition state, increasing the reaction rate.
THE CORE CONCEPT: When an enzyme binds to the reactants (substrates), intermolecular forces like hydrogen bonds, ionic interactions, and van der Waals forces are established. These interactions stabilize partial charges and shift electron density, which helps partially weaken existing bonds and favor new bond formation, forming the high-energy, unstable transition state. The binding energy released during these interactions contributes to lowering the activation energy (AE), making it easier for the reactants to reach the transition state.
Everything else (orienting substrates, stressing bonds, proper alignment) just helps the enzyme use this binding energy more efficiently, but the fundamental reason AE drops is the binding energy.
So the whole mechanism is: When the enzyme binds to the substrate, intermolecular forces are formed that pull electrons toward the active site, partially weakening existing bonds and favoring new ones. This forms the high-energy, unstable transition state. The energy released from these interactions helps lower the energy of the transition state, reducing the activation energy and allowing the reaction to proceed more easily.